Description
What is Repatha (evolocumab) for?
Repatha (evolocumab) is a PCSK9 (proprotein convertase subtilisin kexin type 9) inhibitor antibody for lowering levels of fats in the blood. It is indicated to treat:[1]
- adults with cardiovascular disease to reduce the risk of heart attack, stroke, and certain types of heart surgery.
- adults with high blood cholesterol levels called primary hyperlipidemia (including heterozygous familial hypercholesterolemia [HeFH]) to reduce low density lipoprotein (LDL) or bad cholesterol. It is used along with diet alone or together with other cholesterol-lowering medicines.
- people with a type of high cholesterol called homozygous familial hypercholesterolemia (HoFH), who need additional lowering of LDL cholesterol. It is used along with other LDL-lowering medicines.
It is not known if this medicine is safe and effective in children with HoFH below 13 years of age or in children who do not have HoFH.[1]
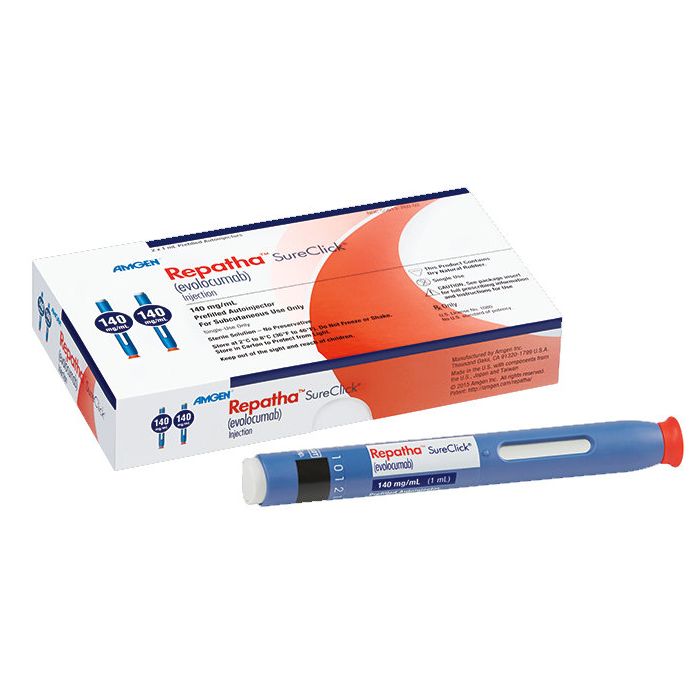
It is available as a solution for injection in pre-filled syringes (140 mg), pre-filled pens (140 mg) and cartridges (420 mg). The cartridges are used together with an automated dosing device called a mini-doser.[1]
How does Repatha (evolocumab) work?
Evolocumab, the active substance in Repatha, is a monoclonal antibody (a type of protein) that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 can bind to cholesterol receptors that are found on the surface of liver cells. This permits these receptors to be broken down inside the cells and allows them to recycle back to the liver cell surface.
By doing so, Repatha can increase the number of receptors on the cell surface. As a result, the receptors can attach to LDL (“bad”)-cholesterol and remove it from the bloodstream. This helps lower blood cholesterol levels.
In patients with mixed dyslipidaemia, Repatha can help to lower other fatty substances from blood.
Where has Repatha (evolocumab) been approved?
Repatha (evolocumab) was approved by:
- The Food and Drug Administration (FDA), USA:
- on August 27, 2015 to treat adults with HeFH, HoFH, or cardiovascular disease.
- on December 1, 2017 to prevent heart attack and stroke.
- The European Medicines Agency (EMA):
- on July 17, 2015 to treat adults with HeFH, HoFH, or cardiovascular disease.
- Health Canada:
- on September 15, 2015 to treat adults with HeFH, HoFH, or cardiovascular disease.
- on June 19, 2018 to prevent heart attack and stroke.
- Pharmaceuticals and Medical Devices Agency (PMDA), Japan:
- on January 21, 2016 to treat adults with HeFH, HoFH, or cardiovascular disease.
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Repatha (evolocumab) taken?
The standard dosage is:[1]
- For adults with primary hypercholesterolaemia, mixed dyslipidaemia and atherosclerotic heart disease:
- 140 mg every two weeks, or
- 420 mg (3 pre-filled syringes or 1 cartridge) once a month.
- For adults and children aged 12 years and older with HoFH:
- 420 mg once a month.
- After 12 weeks, your treating doctor may decide to increase the dose to 420 mg every two weeks.
Inject the medicine of a prefilled pen or syringe under the skin (subcutaneous) into the thigh, upper arm, or stomach (abdomen).[1]
It it used in combination with standard of care treatment.[1]
Reviews
There are no reviews yet.